Damage to the brain resulting from 'asphyxia' at or around the time of birth is an important cause of death and disability among surviving babies. The incidence of 'birth asphyxia' is reported to be much higher in developing countries(1), and presents a formidable challenge to health professionals from the point of view of preventative as well as therapeutic interventions. Several important insights have been gained in the last several years about the epidemiology, diagnosis, and mechanism of brain injury in affected babies but the medical management still remains stereotyped and mostly tends to be regimental, aiming to be neuro-protective. This is based on strategies largely derived from' animal models of hypoxic-ischemic brain injury, and still remains to be tested in controlled clinical trials in human babies. Furthermore, a variety of newer 'designer' treatment based on the concepts of biochemical neurotoxic cascade such as glutamate and free radicals, and accelerated programmed cell death (apoptosis) is currently being proposed for early intervention but it is largely investigational and cannot be recommended for routine clinical use as yet(2).
Birth asphyxia has varying effects on the neonatal brain depending upon the gestational age of the baby and the severity and time of onset of the asphyxiating event(s) which can occur at any point in the infant's antepartum, intrapartum, and postpartum life. Although, hypoxic-ischemic encephalopathy (HIE) is the hallmark of severe asphyxia, such cases can often exhibit multisystem failure involving the heart, kidneys and gastrointestinal systems. This in itself may pose difficult problems whereby optimal treatment of one sys- tem, e.g., volume expansion, may adversely affect another, e.g., acute renal failure. Hence care must be taken to provide general supportive measures while avoiding further insult to an . already compromised system. For this, close monitoring is imperative. The overall management of 'asphyxiated babies' can be categorized into:
These are well described in standard text-books and review articles(3-7). It is beyond the scope of this paper to revisit many of these 'standard' protocols. Instead, the purpose of this paper is to highlight certain aspects of the medical management of birth asphyxia and share some of our experiences with the hope that this will lead to further discussion and evaluation of such practices in individual units. This may also provide a stimulus for clinicians to draw up a consensus protocol in their own unit in order to achieve some consistency in the treatment plan.
Delivery Room Management Although some babies (a small proportion) are born unexpectedly 'flat', it is important to anticipate which infants are at risk of needing resuscitation. In this respect the antepartum and intrapartum history can often be of help. As soon as the infant is delivered, the attending personnel should make a preliminary assessment of the seriousness of the situation. For those requiring tesuscitation, we place firm emphasis on maintenance of the Airway by correct positioning, and establishment of Breathing. The adequacy of inflation is judged by observation of chest wall movement rather than listening for the breath sounds which can be misleading. Once confident of good ventilation, the decision to intubate is only made either if prolonged resuscitation is anticipated or to achieve optimum control of the airway. Most term asphyxiated infants, if adequately ventilated, even in room air, will recover. High concentrations of supplemental oxygen. (80%-100%) are usually recommended in most textbooks and guidelines dealing with newborn resuscitation. There is, however, 'no scientific basis for such recommendation and results from a recent large multicenter international study have even suggested that resuscitation with high oxygen concentration could have detrimental effects on newborn babies(8). In our experience, most such infants do not need supplemental oxygen. Only if room air resuscitation is not successful in the course of 90 seconds do we give concentrations of 40%-60% oxygen and adjust according to clinical response. When intubating, an endotracheal tube (ETT) of the maximum internal diameter which will easily pass the cricoid junction should be used. An important reason for failure of ventilation is a tube of too narrow a diameter allowing the air to flow back through the path of least resistance ('tube' leak) pre- venting adequate expansion of the lungs. This impairs gaseous exchange and further worsens the effect of asphyxia. Presence of thick meconium stained liquor at the time of delivery presents a special situation(9). There is no evidence that elective suctioning of babies' airways as they are being born, or cricoid pressure and epiglottal blockage to prevent aspiration, or thorax compression to prevent spontaneous breathing has any beneficial effect. In fact, these manoeuvres may be potentially harmful causing trauma, vagal stimulation, or induction of deep inspiration, and management of individual babies depends on initial assessment. Crying, implies a partially clear airway and such babies do not usually require any intervention. On the other hand, if still depressed and needing positive pressure ventilation, the oropharynx should be examined, and suctioned gently under direct vision. Babies failing to respond to suction or those who are initially vigorous but rapidly develop respiratory distress should be intubated, any meconium sucked out through the ET tube and ventilation continued. We do not routinely use the intraosseous route in neonatal resuscitation as, contrary to some reports, we find vascular access with an umbilical venous catheter (UVC) rarely fails. Volume expansion during acute resuscitation is only indicated when there are unmistakable signs of shock with evidence of acute blood loss, including feto maternal hemorrhage. The optimal treatment is unclear but usually 10 to 20 ml/kg of plasma expander provides adequate resuscitation and if there is a cIear evidence of volume loss, this should be substituted with 10 ml/kg of 'type specific' blood. Isotonic saline is as effective as 5%albu- min for treating hypotension or as volume re- placement, and has the additicmal advantage of causing less fluid retention in the first 48 hours(10). Review of current literature, in fact, does not support the continued use of colloids for volume replacement in critically ill patients(11 ). Drugs are not routinely used for resuscitation but in case of persisting bradycardia or undetectable output, adrenaline is given through the UVC in a dose of 10 micrograms/ kg (0.1 ml/kg of 1:10,000) and repeated at 3 minute intervals using increasing doses of between 30 to 100 mcg (0.3 to 1 ml/kg), if required. We do not use the endotracheal route for routine administration of adrenaline because of its unproven value in newborn resuscitation when the lungs are filled with fluid. Sodium bicarbonate is used as a 'kick start' if there has been prolonged cardiac arrest not responding to other therapies, in a dose of 1-2 mEq/kg and only given via the UVC. There is no evidence that atropine or calcium have any beneficial effect during the acute phase of resuscitation. Naloxone administration (100 mcg/kg) should be restricted to infants who are considered to have respiratory depression from maternal narcotics and should not be given until adequate ventilation has been established. Babies requiring pro- longed resuscitation should be closely monitored for hypoglycemia and treated initially with 10% dextrose in doses of 2.5 ml/kg. If, after 20-30 minutes of adequate 'A, B, e and D' of resuscitation, the infant still does not show any spontaneous breathing, and the heart rate remains below 60 beats per minute, we consider it appropriate to stop further resuscitation. Further Management Even when the infant is not in need of artificial ventilation following resuscitation, babies who have required intensive resuscitation are often brought to the intensive care unit for further monitoring and close follow up for evidence of any systemic impairment. Neurological: Recognition of neonatal hypoxic ischemic encephalopathy (HIE) re- quires careful observation and examination as the spectrum of signs which may be found in HIE . varies with the severity of the insult. Signs are mainly characterized by abnormalities of tone, altered consciousness, and seizures which may occur in up to 70% of infants with moderate to severe HIE by the end of the first day of life. Potential benefit of preventing further neonatal injury associated with seizures following asphyxia has promoted the widespread use of anticonvulsants, barbiturates in particular, for the prevention of seizures. However, at the present time, there is no scientific data to support this (12), and anticonvulsant therapy to term infants in the immediate period following perinatal asphyxia cannot be recommended, other than in the treatment of prolonged or frequent clinical seizures. Treatment of seizures can often be difficult and may require multiple anticonvulsant therapy. Controversy remains over the most appropriate therapy but we commonly use the following drugs(13): Phenobarbitone: This is used as a first line anticonvulsant for the control of neonatal seizures, given as an initial loading dose of 20 mg/kg IV in order to ensure optimal therapeutic serum concentration. If adequate Control is not achieved, a further dose (half of the loading dose) should be repeated within half an hour. Being a long acting drug, effective therapeutic concentration is maintained for up to 4-5, days, it is frequently unnecessary to continue maintenance treatment. Phenytoin: Given in a loading dose of 20 mg/kg IV over at least 20 minutes, if phenobarbitone fails to control seizures. Clonazepam: This is also a good second line anticonvulsant and is increasingly used in most units. The loading dose is 100 mcg/kg IV as a slow bolus, and babies usually require a further infusion of 10-60 mcg/kg/h aiming for a plasma concentration of 30-100 mcg/litre. Airway stability must be closely monitored as respiratory depression may necessitate ventilatory support. Lignocaine: This drug has recently been advocated for severe neonatal seizures. It is less sedative than other second line drugs and is useful for resistant seizure activity. The loading dose is 2 mg/kg IV followed by 2 mg/ kg/hour by continuous intravenous infusion until seizures are controlled. The infusion should then be weaned over 24-36 hours. In situations where seizure activity may be masked, as in the case of neuromuscular paralysis during ventilation, and facilities for cerebral monitoring do not exist, we electively give a loading dose of phenobarbitone. Treatment of cerebral edema in the asphyxiated neonate remains an area of controversy and speculation. Osmotic agents such as mannitol are not routinely recommended. The use of steroids in cytotoxic edema may be appealing on theoretical grounds, but there is no proof of their effectiveness and they should not be used. High levels of blood PC02 may cause vaso-dilatation of cerebral blood vessels thus contributing to cerebral edema and loss of auto-regulation. Hence it is necessary to maintain PC02 in normal range of 4.5 - 6 kpa (25- 35 mmHg). On the other hand over-ventilation leading to hypocapnia (PC02 <4 kpa) must be avoided as this may be counterproductive by way of producing cerebral vaso- constriction which can exacerbate ischemia. We do not routinely impose fluid restriction in order to 'dry out' the brain unless there is evidence of SIADH, which should be looked for by checking the osmolality of serum and urine simultaneously. Unnecessary fluid restriction in an already hypovolemic infant may further compromise the cardiovascular adequacy. Cardiovascular A common accompaniment to birth asphyxia is myocardial ischemia leading to cardiac dysfunction and systemic hypo- tension. This leads to under-perfusion of peripheral tissues and vital organ systems, i.e., 'shock', thus causing the already compromised brain to be further under-perfused. Clinical signs of cardiac dysfunction and peripheral systemic under perfusion include delayed capillary refill, bradyarrhythmias and increased core-peripheral temperature gradient. Hypotension is a late sign of under- perfusion. The cause of hypotension is rarely blood loss and is more often due to peripheral vaso-dilatation or poor cardiac output. If available, echocardiography provides a useful assessment of cardiac function. In our practice, even if the infant is normotensive, we routinely obtain a chest X-ray to check for cardiomegaly and an echocardiogram to check for evidence of cardiac dysfunction (reduced fractional shortening (normal ~25%), paradoxical septal movement, reduced stroke volume). If there is evidence of global ventricular dysfunction, we start treatment with a combination of dopamine and dobutamine in low doses (each up to 5 mcglkg/minute), titrated if necessary against the response (up to 10-15 mcg/kg/minute). Dopamine is a more effective agent if an increase in systemic blood pressure is required; however, if cardiac output is poor, dopamine will cause a reduction in tissue perfusion. Dobutamine is used in order to bring about an increase in cardiac output. Both dopamine and dobutamine are inactivated by bicarbonate or any alkaline solution, and hence should not be infused through the same line. Epinephrine has been used in cases of post-asphyxial cardiac failure refractory to other inotropes (0.1 mcg/kgl minute-up to a maximum of 1.5 mcg/kgl minute) but it is increasingly felt that such a requirement identifies individual babies as profoundly asphyxiated and raises the question of the appropriateness of continuing treatment. Exuberant use of volume expansion may be detrimental and should be discouraged unless there is evidence of volume depletion. Monitoring of central venous pressure (CVP) would be ideal in such cases but it's routine use is hampered by technical difficulties in the newborn population. As there is no other measurement of right ventricular preload, central venous pressure (CVP) measurement pro- vides important hemodynamic information. CVP is not measured routinely in neonatology and there is shortage of data. Values over 7-8 mmHg are found in babies with myocardial dysfunction or circulatory failure(14). Measurement of inferior vena cava pressure through umbilical venous catheters provides a good approximation to CVP and can be used for guidance regarding fluid treatment(15). Although, its main use is in trend analysis, single measurements can be of value. Pulmonary Assisted ventilation is often needed in severely asphyxiated babies, as treatment for respiratory failure, and as part of the airway management of accompanying problems such as seizures. If muscle relaxants are used, seizure activity may be missed if detection is dependent upon observation alone. If avail- able, Cerebral Function Monitoring (CFM), and EEG monitoring are useful to this end. If seizure activity is anticipated on the basis of clinical assessment, the infant should be given a loading dose of phenobarbitone intravenously. Excessive distending pressures, i.e., PEEP or PIP should be avoided if possible. Arterial PCO2 should be maintained around 4.5-6.0 kpa and both hyper and hypocarbia should be avoided. High distending pressures cause an increase in intra-thoracic volume and pressure, which can affect cerebral circulation, either directly by adding to intracranial pressure or indirectly by lowering cardiac output secondary to reduced venous return-both can have adverse effects on cerebral perfusion pressure (systemic pressure - intracranial pressure). In some cases respiratory management strategies may need to be adapted to deal with associated conditions such as persistent pulmonary hyper- tension of the newborn. This depends on the options (such as high frequency ventilation [HFV], Nitric oxide [NO], 'ECMO') avail- able to the individual units. Renal Acute tubular necrosis is a common sequel to asphyxia and is recognized by a rising plasma creatinine concentration, or by oliguria, defined as an hourly urine flow of less than 0.5 ml/kg. However, incipient and established renal failure may be differentiated by the fractional excretion of sodium (FENA % = VIP sodium concentration x PIU creatinine concentration x 100, where P = plasma and V = urine). Infants with incipient renal failure typically have an FENA of < 3%, while infants with established renal failure have much higher values. Clinical management, however, is more often determined by the urine flow rate and a useful test is to observe the response to frusemide 1-5 mg/kg, given following a fluid challenge (10-20 ml/kg). In the case of a poor response there is no value in repeating the challenge as frusemide is excreted renally and will remain in the circulation for some time, and many babies with acute renal failure develop fluid bverload(16). In most cases, renal failure resulting from acute tubular necrosis improves with conservative treatment which includes strict management of fluid and electrolyte balance. This requires meticulous calculation of fluid input and output at 12 hourly intervals. Urinary retention is a common occurrence in asphyxiated babies, and such babies need to be catheterized in order to obtain an accurate measure of output. To this any insensible loss of 30-40 ml/kg/day, and any other losses such as gastrointestinal losses are added. Total fluid output should be replaced exactly, ml for ml. Although this can be done using a single intravenous fluid, we prefer to establish two peripheral intravenous lines allowing infusion rates to be titrated against losses. We replace insensible loss with 20% glucose (without sodium). The second infusion can be used to replace other measured fluid losses volume for volume using a fluid type dependent upon the fluid lost. It is essential to measure and not to guess the electrolyte concentrations of the losses. Dilutional hyponatremia is common in oliguric babies given a glucose infusion with little or no sodium. Isonatremic solutions can be tailored by adding 30% sodium chloride (5 mmol/ml) or 8.4% sodium bicarbonate (1 mmol/ml). If these measures are inadequate to control hyperkalemia, there are a few non- dialysis options that are safe and effective. The benefits of sodium-potassium ion exchange resins may be short lived, or complicated by sodium overload. . Insulin and glucose infusions have been advocated, but this can cause profound hypoglycemia. β2 adrenoreceptor agonists (Salbutamol) reduce hyperkalemia but unlike in older children, their efficacy is not validated in the newborn. The cardiotoxic effects of hyperkalemia may be reduced by correcting the plasma calcium and magnesium concentrations. Peritoneal dialysis, hemodialysis and hemofiltration are the options available when conservative management fails and the situa- tion is life threatening. The choice of modality will depend upon local expertise and preference; but the major advantages of peritoneal dialysis (PD) are the relatively easy access and its technical simplicity. PD can be man- aged by most special care nurseries with direct supervision from a pediatric nephrologist. Peritonitis is the most significant risk and effluent fluid should be examined twice daily by microscopy (with a differential cell count and microbiological culture where infection is suspected, WCC >50/mm3). Intraperitoneal antibiotics should be added at the earliest suspicion of infection. Enthusiasm for using such techniques however should be carefully balanced against ethical considerations especially in presence of established extensive brain damage. These considerations should be taken into account in line with the institution's 'local ethics policy'. Disseminated Intravascular Coagulation This is a well recognized complication of birth asphyxia and requires prompt treatment with platelets, fresh frozen plasma and cryo precipitate. Investigations It is mandatory to perform certain investigations in babies suffering from the effects of birth asphyxia. These fall broadly into two categories helping to diagnose and plan management. Neonatal encephalopathy may arise secondary to a variety of causes and it is essential to exclude these before blaming 'birth asphyxia'. These causes include sepsis, congenital intrauterine infections (TORCH), inborn errors of metabolism and congenital malformations of the brain. Diagnostic investigations comprise a full septic screen including examination of. all body fluids for evidence of infection, metabolic screening including analysis Of urine for ketones and reducing substances, serum ammonia and plasma lactate levels; and urine and serum amino acid chromatography. Use of neuroimaging is increasingly being considered as a 'gold standard' in babies whose clinical presentation includes neurological dysfunction( 17). For this, cranial ultrasound examination of brain may help during the early neonatal period but CT and MRI brain imaging at 2 to 4 weeks of age after birth appear to pro- vide more useful information. By delineating the underlying neuropathology, such investigations may be useful for prognostication, and provide insight into the timing and nature of the asphyxial insult. Day to day baseline investigations should include serum electrolytes, liver and renal function tests, coagulation studies and arterial blood gases. Because of multisystem involvement in babies with severe birth asphyxia, it only helps to seek advice from other specialist colleagues. Neurological Rehabilitation This should be an integral part of the management of such babies in order that an early and effective plan can be formulated for on- going care(l8). Neurological abnormalities should always be routinely sought as part of neonatal care in babies who survive significant HIE. Persistent generalized disturbances of tone, seizures, continued irritability or decreased alertness, persistent asymmetry of posture and movement, and delay in establishing efficient feeding are all indicative of neurological abnormalities of the infant beyond the neonatal period. The provision of appropriate physiotherapy programmes forms the mainstay of treatment for such babies and sooner or later, it is usual for the speech therapist to be incorporated into their management plan as they have expertise in the management of feeding problems. Thereafter parental sup- port and counselling, treatment of reversible conditions, such as seizures, and plans to limit secondary developmental difficulties, for example, learning and play opportunities, and exercises to prevent the development of contractures, become an essential part of the ongoing multi disciplinary plan, often overseen by a pediatrician who has a special interest in neurodevelopmental pediatrics. |
Thursday, 13 February 2014
Management Of Birth Asphyxia
Tuesday, 4 February 2014
Tachycardia
Tachycardia
Tachycardia is a heart rate of more than 100 beats per minute. The heart normally beats at a rate of 60 to 100 times per minute, and the pulse (felt at the wrist, neck or elsewhere) matches the contractions of the heart's ventricles, the heart's two powerful lower chambers.
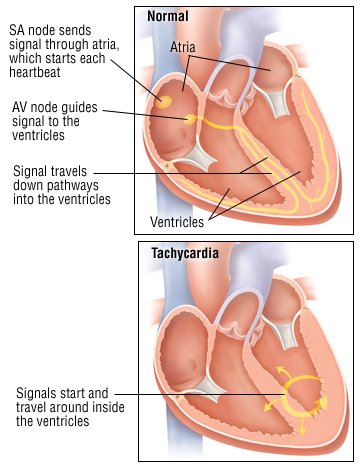 |
Tachycardia can be part of the body's normal response to anxiety, fever, rapid blood loss or strenuous exercise. It also can be caused by medical problems, such as an abnormally high level of thyroid hormones, called hyperthyroidism. In some people, tachycardia is the result of a cardiac arrhythmia (a heart-generated abnormality of heart rate or rhythm). Tachycardia can also be caused by lung problems, such as pneumonia or a blood clot in one of the lung's arteries.
In other cases, tachycardia can be a side effect of some foods and drinks, including coffee, tea, alcohol and chocolate; tobacco; or medication.
Symptoms
Symptoms of tachycardia can include:
- Dizziness, lightheadedness and fainting
- Fatigue (an abnormally tired feeling)
- Palpitations (awareness of a rapid heartbeat)
- Breathlessness
If tachycardia is caused by a medical illness, there will be additional symptoms that are specific to that illness. For example, people who have tachycardia caused by hyperthyroidism also can experience nervousness, insomnia, sweating, tremors and other symptoms related to high levels of thyroid hormones. Tachycardia caused by heart or lung disease often is accompanied by chest pain or shortness of breath or lightheadedness.
Diagnosis
Your doctor will ask you to describe your symptoms. He or she will review your personal medical history and potential causes of tachycardia, including lung disease, thyroid disorders, and medications. Your doctor will want to know if you have a family history of heart disease and cardiac arrhythmias.
During the physical examination, your doctor will check your heart rate and rhythm. Your doctor also will check for an enlarged heart, for heart murmurs (one sign of a heart valve problem), for abnormal lung sounds and for physical signs of thyroid abnormalities (enlarged thyroid gland, hand tremor and an abnormal protrusion of the eyes).
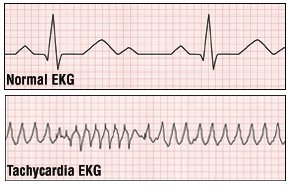
To further evaluate your tachycardia, your doctor will order an electrocardiogram (EKG). However, because some forms of tachycardia come and go, a one-time office EKG may be normal. If this is the case, you may need a test called ambulatory electrocardiography. For this test, you will wear a portable EKG machine called a Holter monitor for a period, usually for 24 hours. If symptoms occur infrequently, you may have to wear a monitor for much longer. You will be taught to press a button to record EKG readings when symptoms occur.
 |
Depending on the results of your physical examination, other tests may be necessary, such as blood tests to measure your red blood cell count and levels of thyroid hormones and an echocardiogram to see if there are any structural abnormalities of your heart. Sometimes, physicians do "electrophysiology testing," in which they insert special catheters within the heart to collect information on the patterns of the heart's electrical activities.
Expected Duration
How long tachycardia lasts depends on its cause. For example, tachycardia resulting from fever will go away when body temperature returns to normal. Tachycardia resulting from blood loss will end when the patient is stabilized with intravenous (IV) fluids and/or blood transfusions. Tachycardia resulting from hyperthyroidism or an adrenal gland tumor will go away when the disorder is treated. Tachycardia caused by medications or diet will go away quickly, usually within hours, when the chemical that is causing the problem is used up by the body or excreted in the urine. Tachycardia caused by cardiac problems can last a long time.
Prevention
Since tachycardia is usually a sign of some underlying medical problem, discovering and treating the cause is the best way to prevent recurrent tachycardia.
The first episode of an arrhythmia that causes a rapid heart beat usually cannot be prevented.
Treatment
The treatment of tachycardia depends on its cause. For example:
- Fever. Fever-related tachycardia can be treated with fever-reducing medications, such as acetaminophen (Tylenol) or ibuprofen (Advil, Motrin and others). If the fever is caused by a bacterial infection, antibiotics also may be needed.
- Blood loss. To treat blood loss, the patient first is stabilized with fluids given intravenously (into a vein) or blood transfusions. Then, the source of the bleeding is found and stitched, or corrected with surgery.
- Hyperthyroidism. Hyperthyroidism can be treated with antithyroid medications such as methimazole (Tapazole, generic versions). Alternative treatments include radioactive iodine, which destroys the thyroid with radiation, or removing most of the thyroid gland with a surgical procedure called subtotal thyroidectomy.
- Cardiac arrhythmias. The treatment depends on the cause of the arrhythmia. In some people, massaging the carotid sinus in the neck will stop the problem. Other people require medications such as digitalis (Lanoxin), beta-blockers, calcium channel blockers, or amiodarone (Cordarone, Pacerone, generic versions). Some patients respond only to radiofrequency catheter ablation, a procedure that destroys the area of abnormal heart tissue that is triggering the tachycardia. Other patients can be treated with electrical cardioversion, a procedure that delivers a timed electrical shock to the heart to restore normal heart rhythm.
- Lung disease. If the tachycardia is caused by a blood clot in the lungs, the usual treatment is medications that dissolve the clot and keep more clots from forming. Pneumonia or other lung problems can be treated with medications for those conditions.
Subscribe to:
Posts (Atom)

.jpg)